Activity
Mon
Wed
Fri
Sun
Jan
Feb
Mar
Apr
May
Jun
Jul
Aug
Sep
Oct
Nov
What is this?
Less
More
Memberships
Biohacking Club
18 members • $5/month
1 contribution to Biohacking Club
For premature babies, the artificial womb seems to be the solution... but it presents us with immense dilemmas ///. Pour les bébé prématurés, l'utérus artificiel semble être la solution… mais il nous met face à des d'immenses dilemmes
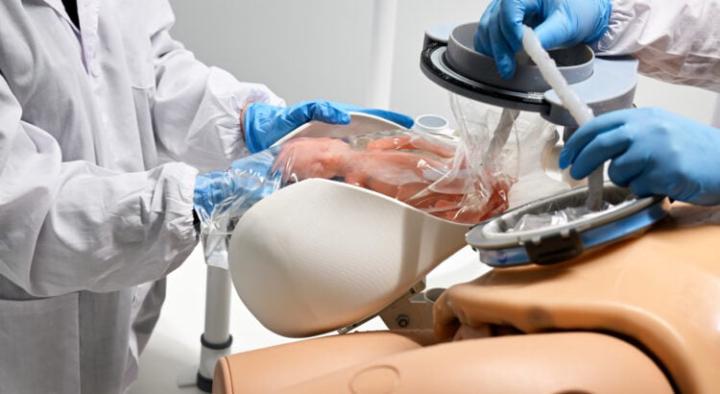
🇺🇲 Officials link: https://www.skool.com/biohacking-1528/classroom/e2e57ff9 The article discusses the spectacular progress of artificial womb technology (AWT), seen as a potential solution to improve survival and reduce complications in extremely premature infants. However, it highlights the major scientific, legal, and ethical questions raised by its application in humans. 1. The Scientific Advance: The Hope of Saving Extremely Premature Infants The concept: AWT aims to simulate the maternal uterine environment after a premature birth. The systems developed (such as the "Ex-Vivo Uterine Environment - EVE" or the "Biobag") often consist of a sterile bag filled with synthetic amniotic fluid, connected to the fetus via the umbilical cord to an extracorporeal oxygenation and nutrition system, replacing the role of the placenta and lungs. Potential benefits: These systems seek to allow fetuses born prematurely (particularly those born at the limits of viability, around 22 to 24 weeks of gestation) to continue a near-normal development, thus reducing the risks of mortality and serious complications (neurological or pulmonary) often associated with incubators and mechanical ventilation. The first trials on lambs have shown very promising results. 2. The Immense Ethical and Legal Dilemmas The article emphasizes that this technology confronts us with dilemmas that go beyond the medical field: Redefining viability and the status of the fetus: If the artificial womb allows a fetus to survive and develop outside the mother's body from as early as 19 or 20 weeks, this could challenge the legal notion of viability and, consequently, the right to abortion. The status of the fetus in this artificial environment becomes a central question: is it a patient or a person in its own right? Risks and informed consent for the mother: The procedure of transferring the fetus to the artificial uterus could require a cesarean section under general anesthesia, carrying risks for the mother. The first clinical trials, if they are conducted, will have to carefully assess the benefits for the baby against the risks for the mother, requiring difficult informed consent.
1-1 of 1
Active 24d ago
Joined Nov 13, 2025


