
Write something
“If stem cell treatments really work…why hasn’t the U.S. approved them yet?”
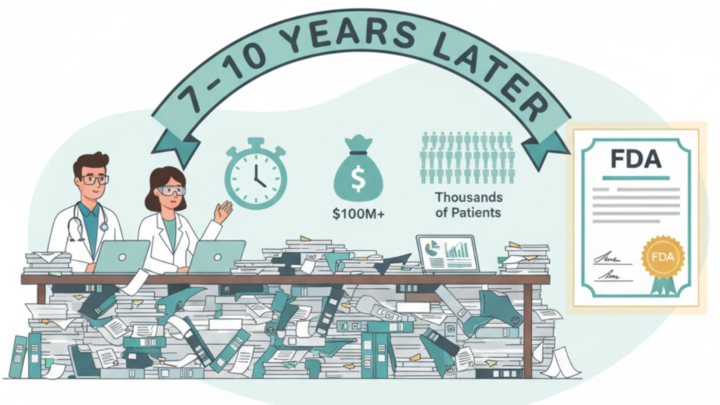
🧭 The Real Answer (In Plain English) It’s not that stem cell therapies don’t work — it’s that the U.S. FDA only approves treatments after a long, expensive process designed for pharmaceutical drugs. Most stem cell therapies today (especially MSCs) do not behave like drugs. They’re living cells, which makes them harder to standardize, package, and patent. To get FDA approval, a company must: ✔ Run multi-phase clinical trials (which cost $100M+ and take 7–10 years) ✔ Prove identical results across thousands of patients ✔ Own the therapy under a commercial license Most regenerative therapies are still in Phase I or II trials, meaning the science is strong, but big companies haven’t completed the approval path yet. 🧪 But Here’s the Catch… The science is already strong in areas like: - Osteoarthritis & joint inflammation - Autoimmune regulation - Graft vs Host Disease (approved in Japan & Europe) That’s why other countries (like Mexico, Japan, Panama, South Korea) allow regulated clinical use of MSCs under medical supervision — even while the U.S. waits for pharmaceutical-style approval. 📌 In Short: The U.S. isn’t saying “stem cells don’t work.” It’s saying: “Come back with a drug-level package.” And stem cells are not simple drugs. 🔍 Reference Jayaram, P. et al. (2025). Ethical and Regulatory Considerations Related to Regenerative Medicine. HSS journal: the musculoskeletal journal of Hospital for Special Surgery.
2
0
1-1 of 1

skool.com/stemcells
I jumped into stem cells blind 🧬 My scientist friend helped me see what’s real 🔬 We made this group so you don’t waste time or money 💡
Powered by

