
Quality Management System for Medical Devices
This course outlines an enriched Quality Management System (QMS). It is designed for medical device manufacturers seeking rigorous compliance with ISO 13485:2016. It also adheres to the European Medical Device Regulation (EU) 2017/745 (MDR), particularly Article 10. The QMS is built on a Process Approach. It integrates core elements, e.g. Risk Management (ISO 14971) and Design Controls. It also includes mandatory Post-Market Surveillance (PMS) and Vigilance systems required by the MDR.
0%

Management Responsibilities
The objective of this course is to provide a brief overview of what the ISO 13485:2016, FDA's QMSR and European (EU) 2017/745 norm and regulations expect from Management Responsibilities
0%

Quality Manual Content under MDR (EU) 2017/745
Description of the content of a Quality Manual for Medical Device Supply Chain
0%

Design Control for Medical Devices
This course establishes a comprehensive, audit-proof framework for Design and Development (D&D) by demonstrating the complete convergence of the MDR's legal mandates with the structured development life cycle defined by ISO 13485:2016 Clause 7.3 (Design and Development). The MDR Annex I General Safety and Performance Requirements (GSPR) function as the ultimate design input, defining device attributes related to safety, performance, and risk management
0%
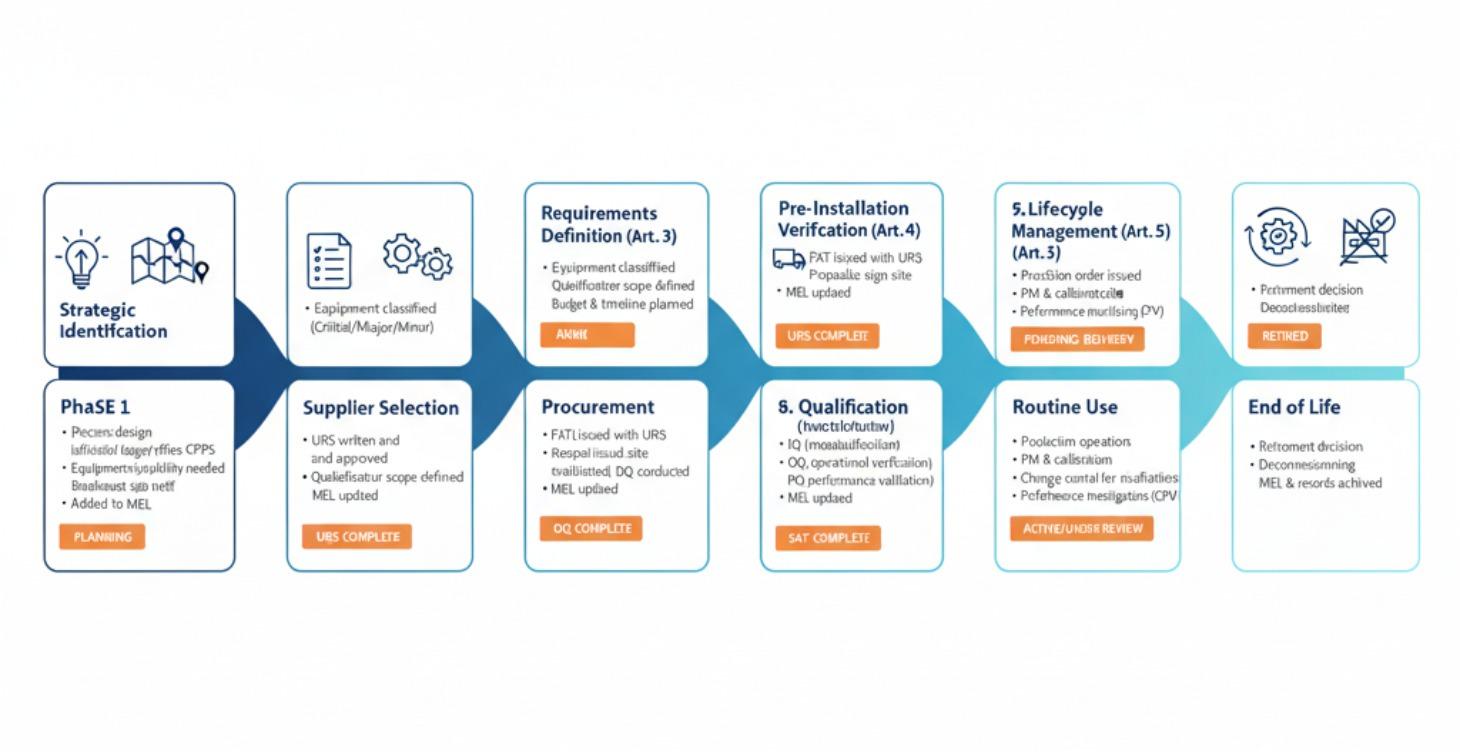
Equipment Qualification and Process Validation
This five article series provides a complete, audit-proof framework for equipment qualification and process validation in medical device manufacturing, fully compliant with:
● EU MDR (EU) 2017/745 - Articles 10, 61, Annex I (GSPR), Annex II (Technical Documentation)
● ISO 13485:2016 - Clauses 7.3 (Design Control), 7.5 (Production), 7.6 (Monitoring Equipment)
● FDA Process Validation Guidance (2011) - Three-stage lifecycle approach
● EU GMP Annex 15 - Qualification and validation principles
0%
1-5 of 5